Biotecnologia
Toxicity of the goji berry fruit associated with artificial excipients and dried without additives
Toxicidade do fruto goji berry associado a excipientes artificiais e desidratado sem aditivos
Toxicity of the goji berry fruit associated with artificial excipients and dried without additives
Acta Scientiarum. Biological Sciences, vol. 40, pp. 1-7, 2018
Universidade Estadual de Maringá

This work is licensed under Creative Commons Attribution 4.0 International.
Received: 26 June 2017
Accepted: 02 May 2018
Abstract: The aim of this study was to evaluate the cytotoxic and genotoxic potential of goji berry fruit-based pharmaceutical powders obtained from three pharmaceutical laboratories. The product A was tested at concentrations of 0.012; 0.025 and 0.05 g mL-1, and B and C at concentrations 0.02; 0.04 and 0.08 g mL-1. It was also evaluated the tea of the dried goji berry fruit (non-additives) in the concentrations 0.035; 0.07 and 0.14 g mL-1 for comparison to the results obtained with powdered goji berry. Tea concentrations in the two exposure times did not cause inhibition of cell division nor cellular alterations to meristem tissues. For the industrialized goji products, all concentrations analyzed caused significant antiproliferative effect to the tissues evaluated at the shortest time of analysis. There were no significant cellular changes in tissues exposed to industrialized goji. Therefore, under the conditions of analysis, goji berry powder, at the three concentrations evaluated, was cytotoxic to root meristems.
Keywords: Lycium barbarum L, natural pharmaceutical product, cytotoxicity, genotoxicity, meristematic tissue.
Resumo: Objetivou-se na presente pesquisa avaliar, em células meristemáticas de raízes de A. cepa, nos tempos de exposição 24 e 48 horas, o potencial citotóxico e genotóxico de produtos farmacêuticos do fruto goji berry em pó, provenientes de três laboratórios farmacêuticos. O produto A foi avaliado nas concentrações 0,012; 0,025 e 0,05 g mL-1, e B e C nas concentrações 0,02; 0,04 e 0,08 g mL-1. Avaliou-se também o chá do fruto seco de goji (não aditivado), nas concentrações 0,035; 0,07 e 0,14 g mL-1, para comparação com os resultados obtidos do fruto em pó. Verificou-se que o chá nas concentrações avaliadas, nos dois tempos de exposição estabelecidos, não ocasionaram inibição da divisão celular e nem alterações celulares aos meristemas de raízes. Para os goji industrializados, todas as concentrações analisadas causaram efeito antiproliferativo significativo aos tecidos avaliados logo no menor tempo de análise considerado. Nenhum dos produtos industrializados causou número significativo de alterações aos meristemas analisados. Assim, os goji em pó foram citotóxicos ao bioensaio utilizado por terem acarretado relevante instabilidade genética aos meristemas de raízes.
Palavras-chave: Lycium barbarum L, produto farmacêutico natural, citotoxicidade, genotoxicidade, tecido meristemático.
Introduction
Lycium barbarum L. popularly known as goji berry or goji, is a shrub Solanaceae, two to four meters high, widely cultivated in China and the Himalayas. Its fruits, also commonly referred to as goji berry and goji, are exported to all continents as spices and mainly for medicinal purposes, because they contain high concentrations of flavonoids and relevant antioxidant potential. They are also sources of an analog of ascorbic acid, 2-O-β-D-Glucopyranosyl acid or AA2βG, as well as, of the carotenoid zeaxanthin (Nascimento et al., 2016) and polysaccharides, which are efficient antioxidant, hypoglycemic and anxiolytic (Amagase & Farnsworth, 2011; Nascimento et al., 2016).
Moreover, since the beginning of this decade, goji fruits have been prescribed by medical doctors and nutritionists, and therefore widely marketed in drugstores around the world, in the form of industrialized natural powdered pharmaceutical products, as a potent supplement in weight reduction and control (Carnés et al., 2013).
As a standard procedure, pharmaceuticals for internal use, such as goji powder, during industrialization are added with chemical excipients, which are inactive micro-ingredients devoid of therapeutic activities and added intentionally. These ingredients are added for the purpose of making such products palatable and protected from the action of undesirable microorganisms, among other characteristics (Araújo & Borin, 2012; Vasconcelos, Rolim, & Peixoto, 2012). Among the excipient additives extensively used by pharmaceutical laboratories are preservatives, colorants, flavorings, sweeteners, thickeners, emulsifiers and stabilizers (Balbani, Stelzer, & Montovani, 2006; Araújo & Borin, 2012).
However, in their technical regulations, the Agência Nacional de Vigilância Sanitária (ANVISA) and the Codex Alimentarius, declare that the flavoring, sweetening, anti-wetting and acidulant additives, although released for use, raise a number of doubts about their potential cytotoxic, genotoxic and mutagenic effects. It also highlights the relevance and urgency of conducting research to evaluates the cytotoxic and genotoxic effects of products added with artificial microingredients (Brasil, 2007). The adverse effects observed from toxicity assessments are very relevant, since they represent important parameters in the elaboration and/or modification of documents that regulate the use of excipients by the industries (Konishi, Hayashi, & Fukushima, 2013; Bezerra, Malaquias, Sousa, & Peron, 2016; Sales, Santos, Sousa, Silva, & Peron, 2017).
However, in a broad search in the scientific literature, we verified that to date there are no studies evaluating cytotoxicity and genotoxicity of goji berries marketed powdered in industrialized form. In contrast, for fresh goji berry fruit, we found studies that evaluate its toxic potential at the cellular level, such as the work of Sayeed et al. (2017), who evaluated the toxicity of this fruit through in vivo micronucleus test and comet assay using colon cells from Wistar rats and reported that the aqueous goji extract was neither cytotoxic nor genotoxic to the test systems used. In addition, Yang, Zhao, Chen, Chan, and Wu (2015) and Potterat (2010) verified that goji fruit tea did not alter cell division and was antigenotoxic to normal cell lines. Thus, it is relevant to evaluate, through appropriate toxicity bioassays, the cytotoxicity and genotoxicity of the powdered fruit added with excipient compounds, in order to determine if this has toxic potential significantly different from its fresh form. Evaluations like these can contribute to the safe and effective consumption of these industrialized natural products by the population.
Plant bioassays are appropriately sensitive and simple in monitoring the toxic effects at the cellular level of chemical compounds (Caritá & Marin-Morales, 2008; Campos-Ventura, & Marin-Morales, 2016). Among them, the root meristem of Allium cepa L. (onion) is considered in the scientific circle as an efficient bioassay for the initial screening of the genetic toxicity of chemical compounds due to the low number of chromosomes (2n = 16), which favors the detection of mitotic spindle or aneugenic defects, and disturbances in the cell proliferation index (Neves, Ferreira, Lima, & Peron, 2014; Bianchi, Mantovani, & Marin-Morales, 2015). It is a test system accepted internationally by research agencies as an instrument of evaluation with accurate sensitivity to analyze the cytotoxicity and genotoxicity of a substance of interest, since the results obtained often show satisfactory similarity to those obtained through animal testing systems and cell cultures (Herrero et al., 2012; Lacerda, Malaquias, & Peron, 2014; Tabrez et al., 2011; Bianchi et al., 2015; Campos-Ventura et al., 2016; Santana et al., 2016). As an example, we can cite the researches conducted by Gomes, Oliveira, Carvalho, Menezes, and Peron (2013) and Oliveira, Alves, Lima, Sousa, and Peron (2013), which evaluated in root meristem cells of A. cepa the toxic potential of synthetic colorings used in the food and pharmaceutical industries and obtained results similar to those obtained via animal testing systems and via cell cultures.
In this context, the present study aimed to evaluate, in root meristem cells of A. cepa, the cytotoxicity and genotoxicity of natural pharmaceutical products of goji berry fruit powder obtained from three different pharmaceutical laboratories of relevant performance in the Brazilian and international marketing of medicines. The evaluation of dried goji berry, without additives, tea was also performed with the purpose of comparing the results with data observed for the industrialized form considering the same test system.
Material and methods
The dried goji fruit, without addition of artificial excipients, was acquired in an herbal store, in the city of Teresina, State of Piauí, Brazil, specialized in the commercialization of natural products. In the industrialized form, the goji fruit marketed in powder form, was acquired in a unit of a national drugstore network in the municipality of Picos, State of Piauí, Brazil. The industrialized products, from three pharmaceutical laboratories, were discriminated in the present research as A, B and C.
For determination of goji concentrations to be analyzed for toxicity, the form of preparation and ingestion indicated on the labels of each product, fresh and industrialized, was used as the definition parameter. For the preparation of the tea, 70 grams of the dried fruit should be mixed with a liter of boiling water. Thus, three concentrations of the tea were defined for analysis, which were 0.035; 0.07 and 0.14 g mL-1. Regarding the industrialized goji for laboratory A, the suggested concentration was 70 grams fruit powder for 200 mL water. Thus, the analysis concentrations for A were 0.012; 0.025 and 0.05 g mL-1. For laboratories B and C, the ideal concentration suggested for consumption was 5 g of goji powder for 200 mL water, and concentrations for toxicity analysis were 0.012; 0.025 and 0.05 g mL-1. To obtain all the concentrations evaluated in the present study, we used distilled water.
For toxicity analysis, onion bulbs were placed in aerated bottles with distilled water, at room temperature (± 27ºC), to obtain 2.0 cm long roots. For the analysis of each goji sample, an experimental group with five onion bulbs was established. Before placing the roots in contact with their respective goji samples (treatments), some roots were collected and fixed to serve as control of the bulb itself. Then, the remaining roots were placed in contact with their respective treatments for 24 hours, a procedure called 24 hours exposure time. After 24 hours, some roots were collected and fixed. After, the remaining roots of each bulb were returned to their respective treatments, where they remained for additional 24 hours, which was called 48 hours exposure time. Subsequently, roots were again collected and fixed. The 24 and 48 hours exposure times were chosen with the purpose of evaluating the action of goji in more than one cell cycle. Roots were fixed in Carnoy 3: 1 (ethanol: acetic acid) for 24 hours. In each collection, on average, three roots were taken per bulb.
The slides, on average 03 per bulb, were mounted according to Guerra and Souza (2002) and analyzed under an optical microscope (Zeiss brand) using 400x objective lens. For each onion bulb, we analyzed 1,000 cells, totaling 5,000 cells for each control, 24 hours exposure time and 48 hours exposure time of each treatment group analyzed. Thus, for each goji concentration, we analyzed a total of 15,000 cells. Cells were observed in interphase, prophase, metaphase, anaphase and telophase. From this analysis, the mitotic index (MI) was determined by means of the following equation: (total number of cells in mitosis ÷ total number of cells analyzed) x 100. The MI value was the parameter used for the determination of the cytotoxic potential of goji berry in the forms analyzed.
In addition, we examined the genotoxicity of goji by the frequency of cell alterations or mitotic spindle defects, including C-metaphases, Multipolar anaphase, Anaphase and telophase bridges, Gene amplifications, Cells with adhesion, Nuclear buds and Micronuclei. For the statistical analysis of data on cytotoxicity and genotoxicity of the samples, we applied the Chi-square test (χ2), with <0.05 probability level, statistical program Bioestat, version 5.3.
Results and discussion
As described in Table 01, the root meristems exposed to the three concentrations of goji berry tea at the 24 and 48 hours exposure times, when compared to the observed cell division for their respective controls, showed no alteration in their mitotic indices. There was also no significant difference between the mitotic indices obtained for the two exposure times of each tea concentration evaluated. Thus, it can be stated that the evaluated goji teas, under the conditions of study established, caused no cytotoxicity to root meristem cells of A. cepa. In addition, there were no cellular alterations in plant tissues exposed to such solution. These results corroborate the data of non-cytotoxicity and genotoxicity of goji fruit found in the scientific literature (Yang et al., 2015; Potterat, 2010), previously mentioned in this work.
However, the three concentrations of goji referring to PL A (Table 01), at 24 and 48 hours of exposure, significantly reduced the cell division of the meristematic tissue when compared to the mitotic indices obtained for their respective controls. Moreover, for the concentration 0.035 g mL-1 of PL A, mitotic indices obtained for the two exposure times evaluated differed from each other, since in the 48 hours exposure time, the cell division was markedly lower than that observed for the exposure time of 24 hours. However, for PL A at concentrations of 0.07 and 0.14 g mL-1, inhibition of cell division was observed shortly at the 24 hours exposure time. For the three concentrations of goji berry products of PLs B and C (Table 1), the mitotic indices verified for 24 hours exposure time were considered the same as those obtained for their respective 48 hours exposure time.
In view of this, based on the data described in Table 1, there is a difference in the action of fresh goji berry in relation to the industrialized goji as to the toxicity caused, once the excipients added were cytotoxic to the meristem tissue evaluated for causing a marked antiproliferative effect at the lowest concentrations and in the shortest exposure time. It is also noted that all goji concentrations considered as ideal for consumption by the pharmaceutical laboratories by which they were produced have caused significant inhibition of cell proliferation, demonstrating relevant cytotoxic potential.
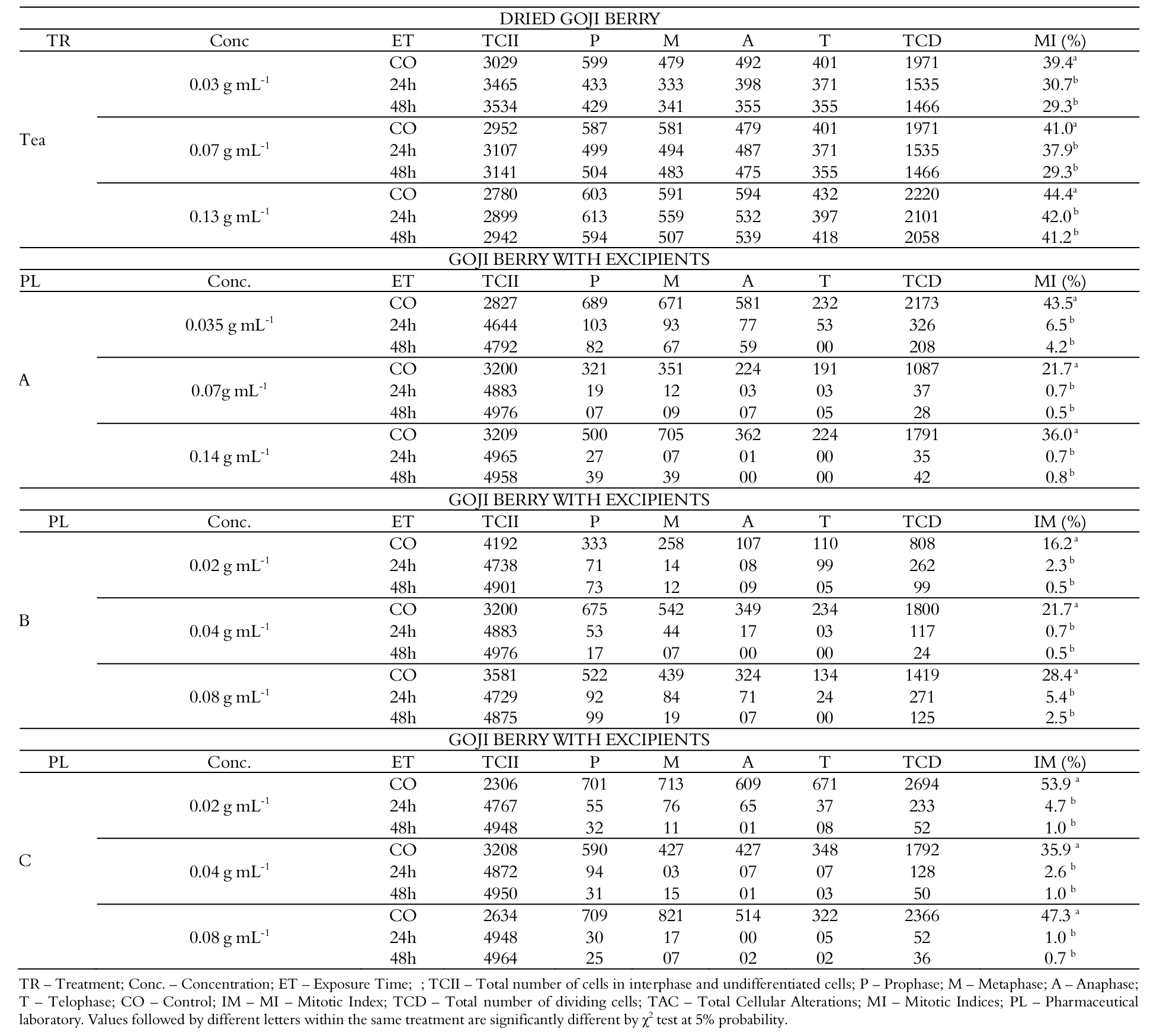
Table 1.
Number of cells observed in each phase of the cell cycle of the root meristem of Allium cepa exposed for 24 and 48 hours to goji berry tea and to industrialized powdered goji berry, of the chemical laboratories A, B and C.
TR – Treatment; Conc. – Concentration; ET – Exposure Time; ; TCII – Total number of cells in interphase and undifferentiated cells; P – Prophase; M – Metaphase; A – Anaphase; T – Telophase; CO – Control; IM – MI – Mitotic Index; TCD – Total number of dividing cells; TAC – Total Cellular Alterations; MI – Mitotic Indices; PL – Pharmaceutical laboratory. Values followed by different letters within the same treatment are significantly different by χ2 test at 5% probability.
According to Caritá and Marin-Morales (2008), significant alterations are triggered when there is a pronounced antiproliferative effect in tissues with intense proliferation and normal metabolic performance - such as the meristem of roots used in the present study - exposed to chemical compounds with potential to cause genetic instability, significantly compromising the growth and functioning of the organs in which they are acting. Furthermore, Gomes et al. (2013); Bezerra et al. (2016) and Carvalho et al. (2016) state that the inhibition of cell proliferation triggered by cytotoxic compounds in tissues of intense cell proliferation and normal functioning and/or without cellular alterations - once again mentioning the root meristems used as bioassays in the present research - is harmful to the organisms by inhibiting or limiting the replacement of cells, modifying the production of proteins and, consequently, resulting in malfunction of the organ or tissue where it is located.
No significant cellular alterations were registered in the meristematic cells exposed to the goji concentrations from PLs A, B and C. Nevertheless, Sales et al. (2017) explain that inhibition of division in normal tissues occurs by the action of agents that affect the integrity of the nuclear spindle during mitosis promoting significant chromosomal derangement. Considering that the principle of the cell cycle is the formation of identical cells, the production of cells with alterations in structure and/or chromosome number make the cellular operation unfeasible and tend to be eliminated from tissues with normal performance, a condition that is suggested to explain the result of a marked antiproliferative effect against the non-significant frequency of cellular alterations observed in the present study.
As aforementioned, natural pharmaceutical products, such as the industrialized goji berries evaluated herein, contain artificial excipients in their formulation. Similarly, a toxicity evaluation of Ginkgo biloba L. leaves in natura and associated excipients in different bioassays showed that the industrialized forms were toxic at the cellular level (manuscript in press). These results corroborate the results obtained in the present study, suggesting that the excipients in the industrialization of natural products have cytotoxic effects.
Results from studies on cellular toxicity of some excipient compounds used in the formulation of pharmaceuticals and food products, according to Brazil (1999), during industrialization will be reported. However, it is important to state that, with the exception of colorings and preservatives, all the microingredients to be reported were insufficiently evaluated, according to ANVISA, regarding their toxic potential at the systemic and cellular levels (Brasil, 2007; Sales et al., 2017).
For colorings, the only authorized for use in pharmaceutical products in general are Twilight Yellow, Tartrazine and Red 40, azo additives because they contain the azo group, a nitro derivative with the property of producing aromatic amine and sulphanilic acid, as well as Ponceau 4R, Erythrosine and the Bright Blue (Gomes et al., 2013; Sardi et al., 2010). These six dyes showed potential in altering the turner-over of the cells during interphase, expressively inhibiting cell division, and the process of regenerative hyperplasia, which contributed significantly to the development of cancers in the digestive tract of rodents (Sardi et al., 2010).
Aspartame, sodium cyclamate, potassium acesulfame and sodium saccharin are among the sweeteners permitted as excipients (Balbani et al., 2006;Vasconcelos et al., 2012). Using cell lines Caco-2 (colon cells), HT-29 (colon cells) and HEK-293 (kidney cells), Van EyK (2015) reported that these sweeteners were cytotoxic and genotoxic to the cells studied. Corroborating with the results of these researchers, Zaineddin et al. (2012), through the comet assay, found that sodium saccharin and sodium cyclamate were genotoxic and mutagenic to rodent colon cells, significantly reducing the cell division of the analyzed tissue.
The anti-wetting agents used in pharmaceuticals are calcium phosphate, silicon dioxide, calcium carbonate and magnesium carbonate (Villanova, Oréfice & Cunha, 2010). There were no studies in the scientific literature evaluating cytotoxicity and genotoxicity for the calcium phosphate and calcium carbonate. In turn, for silicon dioxide, Rajiv et al. (2016) argued that this compound had the property to significantly decrease the cell viability of human lymphocytes in normal cell culture, as well as to promote significant cellular alterations, demonstrating a broad genotoxic potential. For the magnesium carbonate, Ahamed et al. (2015) observed that this chemical had the ability to reduce cellular metabolism in normal human liver cell culture, showing to be significantly cytotoxic.
As for flavorings, the aroma and flavor ingredients used in medicines are only those of fruit (Balbani et al., 2006). Sales et al. (2017) and Moura et al. (2016) evaluated some of these flavorings and verified that such additives had the property of inducing significant damage to the mitotic spindle, and consequently the cell division of human peripheral blood cells, and were genotoxic to mouse blood tissue erythrocytes, significantly inducing the formation of micronucleated cells in the bone marrow of treated animals. In relation to the chemical constituents responsible for retarding the action of microorganisms, enzymes, as well as physical agents in pharmaceuticals, include potassium benzoate, sodium benzoate and potassium nitrate, preservatives which, according to Mpountoukas et al. (2010) and Zeguin, Yüzbaşioğlu, Unal, Yilmaz, & Aksoy (2011), were cytotoxic and genotoxic to normal human peripheral blood cells.
The toxicity results of the mentioned excipients validate those obtained in the present study for the industrialized goji evaluated, as they also caused genetic instability mainly to the cell cycle of the bioassays in which they were evaluated. No studies were found in the literature to evaluate the toxicity at the cellular level of additives of thickening, emulsifying and stabilizing action.
Conclusion
The data obtained in the present study with the meristematic root cells of A. cepa showed that the industrialized goji berries evaluated had a significant potential to cause toxicity to the cells tested at all evaluated concentrations, including those indicated for use by pharmaceutical laboratories.
These results indicate the need to evaluate powdered goji pharmaceuticals in animal testing systems, from treatments with longer exposure times, to verify and deepen the results obtained here.
It is important to emphasize that the results obtained here with respect to genetic instability caused by the action of the industrialized goji are of great relevance since to date there are no toxicity studies involving such pharmaceutical products.
References
Ahamed, M., Alhadlay, H. A., Ahmad, J., Siddiqui, M. A., Khan, S. T., Mussarat, J… Al-Khedhairy, A. A. (2015). Comparative cytotoxicity of dolomite nanoparticles in human larynx HEp2 and liver HepG2 cells. Journal of Applied Toxicology, 35(6), 640-650. doi: 10.1002/jat. 3097
Amagase, H., & Farnsworth, N. R. A. (2011). Review of botanical characteristics, phytochemistry, clinical relevance in efficacy and safety of Lycium barbarum fruit (Goji). Food Research International, 44(7), 1702-1717. doi: 10.1016/j.foodres.2011.03.027
Araujo, A. C. F., & Borin, M. F. (2012). Influência de excipientes farmacêuticos em reações adversas a medicamentos. Brasilia Médica. Brasilia, 49(4), 267-278.
Balbani, A. P. S., Stelzer, L. B., & Montovani, J. C. (2006). Excipientes de medicamentos e as informações da bula. Revista Brasileira de Otorrinolaringologia, 72(1), 400-406. doi: 10.1590/S0034-72992006000300018.
Bezerra, M. D. S., Malaquias, G. O., Sousa, J. M. C., & Peron, A. P. (2016). Cytotoxic and genotoxic potential of powdered juices. Ciência e Tecnologia de Alimentos, 36(1), 49-55. doi: 10.1590/1678-457X.0006
Bianchi, J., Mantovani, M. S., & Marin-Morales, M. S. (2015). Analysis of the genotoxic potential of low concentrations of Malathion on the Allium cepa cells and rat hepatoma tissue culture. Journal Environmental Sciences, 36(1), 102-111. doi: 10.1016/j.jes.2015.03.034
Brasil. Agência Nacional de Vigilância Sanitária [ANVISA]. (2007). Resolucão da diretoria colegiada RDC nº. 5, de 15 de Janeiro de 2007. Brasília: ANVISA. Retrieved on Dec 3, 2016 from www.anvisa.gov.br/legis/resol/2007/rdc/02_ 170107rdc.pdf
Brasil. Agência Nacional de Vigilância Sanitária [ANVISA]. (1999). Resolução n. 17, de 30 de abril de 1999 – ANVISA, 1999. Retrieved on Oct 5, 2016 from www.bvsms.saude.gov.br/bvs/is_digital/is_0407/pdfs/IS27%284%29091.pdf
Campos-Ventura, B., & Marin-Morales, M. A. (2016). Micronuclei and chromosome aberrations derived from the action of Atrazine herbicide in Allium cepa meristematic cells. SDRP Journal of Earth Sciences & Environmental Studies, 1(1), 22-28.
Caritá, R., & Marin-Morales, M. A. (2008). Induction of chromosome aberrations in the Allium cepa test system caused by the exposure of seeds to industrial effluents contaminated with azo dyes. Chemosphere, 72(5), 722-725. doi: 10.1016/j.chemosphere.2008.03.056
Carnés, J., de Larramendi, C. H., Ferrer, A., Huertas, A. J., López-Matas, M. A., Pagán, J. A., ... Peña, M. (2013). Recently introduced foods as new allergenic sources: sensitisation to Goji berries (Lycium barbarum). Food Chemistry, 137(1), 130-135. doi: 10.1016.j.ffodchem. 2002.10.005
Carvalho, F. R., Moura, A. G., Rodrigues, G. F., Nunes, N. M., Lima, D. J., Pessôa, C., ... Peron, A. P. (2016). Are salty liquid food flavorings in vitro antitumor substances? Anais da Academia Brasileira de Ciências, 88(3), 1419-1430. doi: 10.1590/0001-3765201620150553
Donno, D., Beccaro, G. L., Mellano, M. G., Cerutti, A. K., & Bounous, G. (2015). Goji berry fruit (Lycium spp.): antioxidant compound fingerprint and bioactivity evaluation. Journal of Functional Foods, 18(1), 1070-1085. doi: 10.1016/j.jff.2014.05.020
Gomes, K. M. S., Oliveira, M. V. G. A. D., Carvalho, F. R. S., Menezes, C. C., & Peron, A. P. (2013). Citotoxicity of food dyes sunset yellow (E-110), bordeax red (E-123), and tatrazine yellow (E-102) on Allium cepa L. root meristematic cells. Ciência e Tecnologia de Alimentos, 33(1), 218-223. doi: 10.1590/S0101-20612013005000 012
Guerra, M., & Souza, M. J. (2002). Como observar os cromossomos: um guia de técnicas em citogenética vegetal, animal e humana (304p.). Ribeirão Preto, SP: FUNPEC.
Herrero, O., Martín, J. P., Freire, P. F., López, L. C., Peropadre, A., & Hanzen, M. J. (2012). Toxicological evaluation of three contaminant of emerging concern by use of Allium cepa test. Mutation Research, 743(1), 24-34. doi: 10.1016/j.mrgentox.2011.12.028
Konishi, Y., Hayashi, S. M., & Fukushima, S. (2013). Regulatory forum opinion piece*: supporting the need for international harmonization of safety assessments for food flavoring substance. Toxicologic Pathology, 42(6), 949-953. doi: 10.1177/0192623313495603
Lacerda, L. P., Malaquias, G., & Peron, A. P. (2014). Antiproliferative action of aqueous extracts of Hymenaea stigonocarpa Mart. (Fabaceae) on the cell cycle of Allium cepa L. Anais da Academia Brasileira de Ciências, 89(3), 1147-1150. doi: 10.1590/0001-3765201420130163
Mpountoukas, P., Pantazaki, A., Kostareli, E., Christodoulou, P., Kareli, D., Poliliou, S., & Lialiaris T. (2010). Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chemical and Toxicology, 48(10), 2934-2944. doi: 10.1016/j.fct.2010. 07.030
Nascimento, W. M., Souza, L. M. S., Costa, D. D. A. F., Oliveira, R., Monte, S. M., Silva, G. C. E., ... Maia Filho, A. L. M. (2016) Genotoxicity of Goji Berry (Lycium barbarum) in vivo Mammalian Cells. International Journal of Pharmaceutical Science Invention, 5(4), 23-26. doi: 16/j.fct.201
Neves, E. S., Ferreira, P. M. P., Lima, L. H. G. M., & Peron, A. P. (2014). Action of aqueous extracts of Phyllanthus niruri L. (Euphorbiaceae) leaves on meristematic root cells of Allium cepa L. Anais da Academia Brasileira de Ciências, 86(3), 1131-1137. doi: 10.1590/0001-3765201420130107
Oliveira, M. V. A., Alves, D. D. L., Lima, L. H. G. M., Sousa, J. M. C., & Peron, A. P. (2013). Cytotoxicity of erythrosine (E-127), brilliant blue (E-133) and red 40 (E-129) food dyes in a plant test system. Acta Scientiarum. Biological Sciences, 35(4). doi: 10.4025/actascibiol sci.v35i4.18419
Potterat, O. (2010). Goji (Lycium barbarum and Lycium chinense): phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Medica, 76(1), 7-19. doi: 10.1055/s-00291186218
Rajiv, S., Jerobin, J., Saranya, V., Nainawat, M., Sharma, A., Makwana, P., ... Mukherjee, A. (2016). Comparative cytotoxicity and genotoxicity of cobalt (II, III) oxide, iron (III) oxide, silicon dioxide, and aluminum oxide nanoparticles on human lymphocytes in vitro. Human & Experimental Toxicology, 35(2), 170-183. doi: 10.1177/0960327115579208
Sales, I. M. S., Santos, F. K. S., Sousa, J. M. C., Silva, F. C. C., & Peron, A. P. (2017). Acute toxicity of grape, plum and orange synthetic food flavourings evaluated in vivo test systems. Food Technology and Biotechnology, 55(1), 131-137. doi: 10.17113/ftb.55.01. 17.4770
Santana, G. M., Deus, M. S. M., Sousa, J. M. C., Ferreira, P. M. P., Fernandes, H. B., & Peron, A. P. (2016). Antimitotic and antimutagenic action of the Hymenaea stigonocarpa bark on dividing cells. Brazilian Journal of Biology, 76(2), 520-525. doi: 10.1590/1519-6984.23014
Sardi, M., Haldemann, Y., Nordmann, H., Bottex, B., Safford, B., Smith, B., & Jasti, P. R. (2010). Use of retailer fidelity card schemes in the assessment of food additive intake: sunset yellow a case study. Food Additives and Contaminants, 27(11), 1507-1515. doi: 10.1080/19440049.2010.495728
Sayeed, M. A., Bracci, M., Lazzarini, R., Tomasetti, M., Amati, M., Lucarini, G., ... & Santarelli, L. (2017). Use of potential dietary phytochemicals to target miRNA: Promising option for breast cancer prevention and treatment? Journal of Functional Foods, 28(1), 177-193. doi: 10.1016/j.jff.2016.11.008
Tabrez, S., Shakil, S., Urooj, M., Damanhouri, G. A., Abuzenadah, A. M., & Ahmad, M. (2011). Genotoxicity testing and biomarker studies on surface waters: an overview of the techniques and their efficacies. Journal of Environmental Science and Health, Part C, 29(3), 250-275. doi: 10.1080/10590501.2011. 601849
Van Eyk, A. D. (2015). The effect of five artificial sweeteners on Caco-2, HT-29 and HEK-293 cells. Drug and Chemical Toxicology, 38(3), 318-327. doi: 10.3109/01480545.2014.966381
Vasconcelos, P. A. F., Rolim, L. A., & Peixoto, M. S. (2012). Influência dos excipientes multifuncionais no desempenho dos fármacos em formas farmacêuticas. Revista Brasileira de Farmácia, 93(2), 136-145.
Villanova, J. C. O., Oréfice, R. L. & Cunha, A. S. (2010). Aplicações farmacêuticas de polímeros. Polímeros: Ciência e Tecnologia, 20(1), 51-64.
Yang, R. F., Zhao, C., Chen, X., Chan, S. N., & Wu, J. Y. (2015). Chemical properties and bioactivities of Goji (Lycium barbarum) polysaccharides extracted by different methods. Journal of Funcional Foods, 17(1), 903-909. doi: 10.1016/j.jff.2015.06.045
Zaineddin, A. K., Buck, K., Vrieling, A., Heinz, L., FleschJanys, D., Linseisen, J., & Chang-Claude J. (2012). The association between dietary lignans, phytoestrogen-rich foods, and fiber intake and postmenopausal breast cancer risk: a German case-control study. Nutrition Cancer, 64(5), 652-665. doi: 10.1080/01635581.2012.683227
Zeguin, N., Yüzbaşioğlu, D., Unal, F., Yilmaz, S., & Aksoy, H. (2011). The evaluation of the genotoxicity of two food preservatives: sodium benzoate and potassium benzoate. Food Chemical and Toxicology, 49(4), 763-769. doi: 10.1016/j.fct.2010.11.040